Cross-coupling reaction
We utilize a range of cross-coupling reactions that make use of transition metals as catalysts. These include Suzuki-Miyaura coupling and organic boronic acid (ester) derivatives, as well as OLED (organic light-emitting diode) compounds.
1. Suzuki-Miyaura coupling
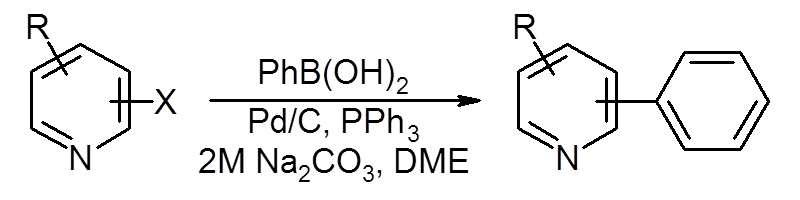
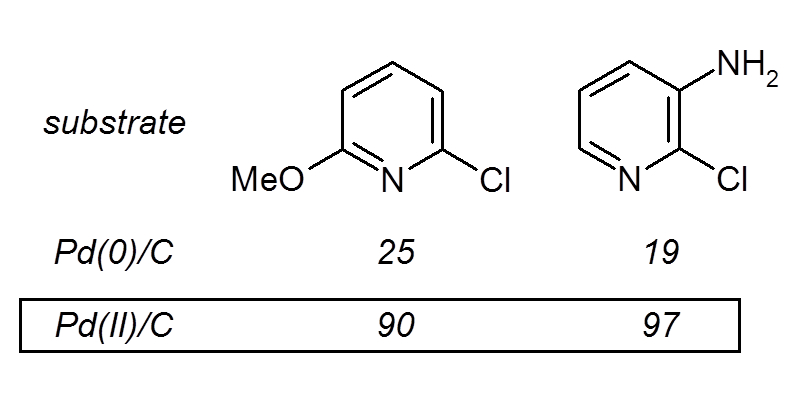
We have developed a new form of heterocyclic coupling making use of divalent paladium (Pd(II)/C) and triphenylphosphine.
This reaction is much more efficient than traditional Pd(0)/C, allowing for creating, among others, phenylpyridines.
Pictured is Prof. SUZUKI Akira, professor emeritus of Hokkaido University (2010 Nobel Prize in Chemistry)

2. Organic boronate ester derivatives
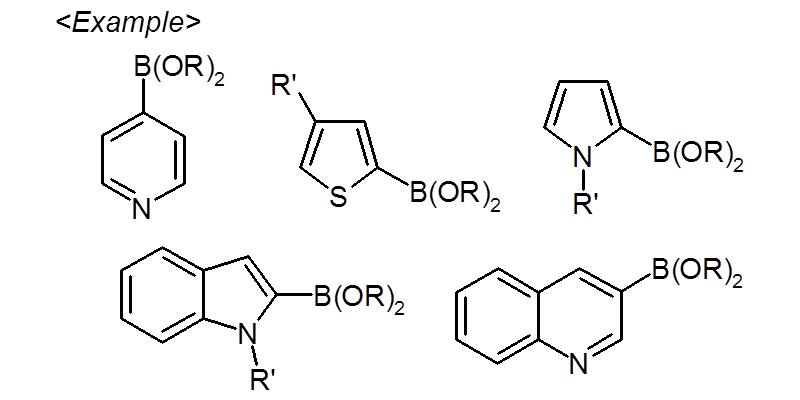
Aromatic and heterocyclic boronic acid (ester) derivatives act as key intermediaries in Suzuki-Miyaura reactions, making them extremely useful compounds in biaryl compound synthesis.
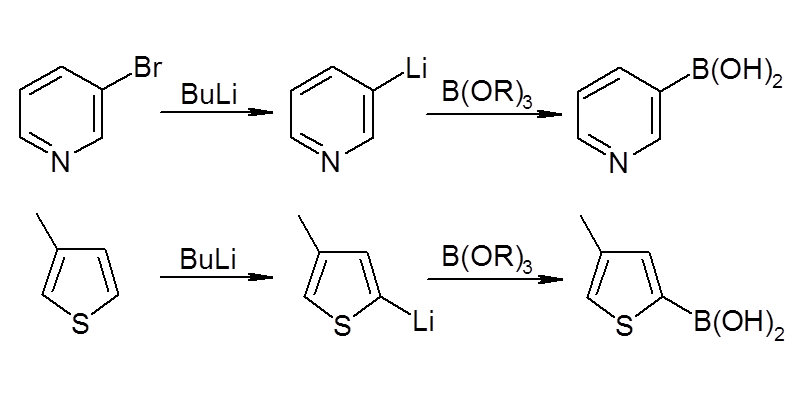
Through boronic acid synthesis using aryllithium compounds and arylmagnesium compounds and direct boronic acid synthesis, we have established a synthetic technology for a wide range of boronic acid (ester) derivatives.
Boronic acid synthesis from lithium and magnesium compounds
We are equipped to provide clients with a range of boronic acid (esters), from lab scale on through to industrial scale.
Ultra low temperature (-60 degrees Celsius) reaction equipment is used for synthesis.
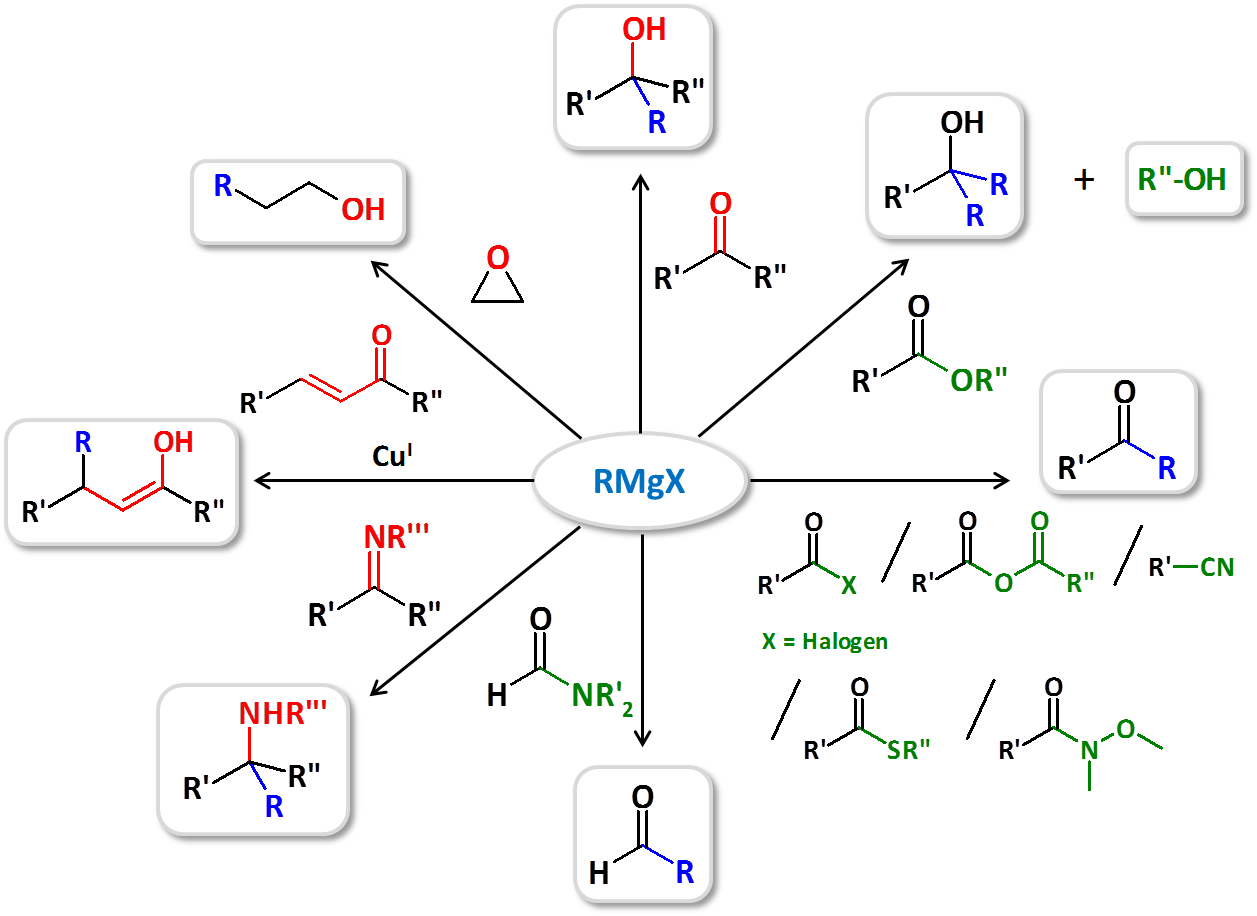
Reactions using Grignard reagents
Our firm manufactures and sells Grignard reagents of a very high purity. We also excel at complex reactions that make use of Grignard reagents and require dry and oxygen-free conditions, allowing us to synthesize a wide range of compounds. Below is a example for some reactions achieved using Grignard reagents.
For product inquiries and to download a catalog,
please click the button below.



